52+ calculate the formal charge on the chlorine cl atom
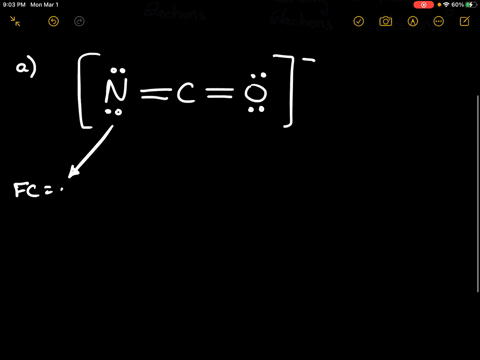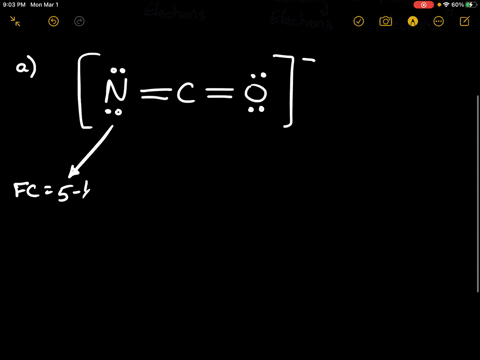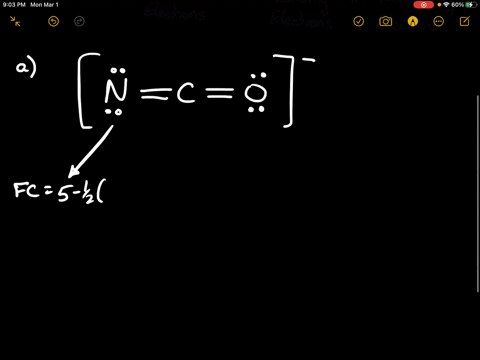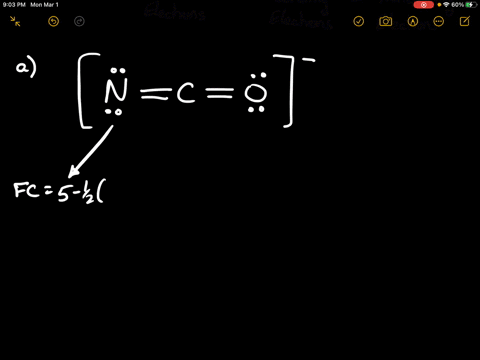
Of valence electrons in the free state of atom - Total no. Web The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms.
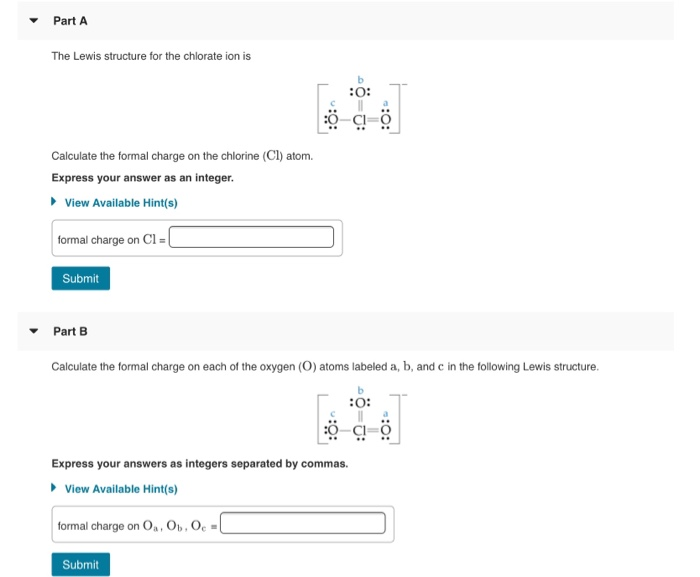
Solved Part A The Lewis Structure For The Chlorate Ion Is Chegg Com
Subtract this number from the number of valence electrons for the neutral atom.

. Web Formal charge is the individual electric charges on the atoms in a given polyatomic molecule. Web Part A The Lewis structure for the chlorate ion is b 0. Web The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms.
Web The formula for the formal charge of an atom is given by. Web What is the shape around chlorine atom in ClO 3-ion. Web In order to calculate the formal charges for ClO2- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding el.
View Available Hints formal. There are three σ bonds and a one lone pair around chlorine atom in lewis structure of ClO 3-ion. The structure with the lowest formal charge on each.
Web In the molecule the charge on Cl is neutral. F C Total no. Solve Study Textbooks Guides.
There are SEVEN valence electrons surrounding the atom and thus with 10 inner core electrons there are 17. Express your answer as an integer. Web Click hereto get an answer to your question Calculate the formal charge on Cl atom in HClO4.
Join Login Class 11 Chemistry. These charges help in knowing if the given structure of the. Web The Formal Charge is a somewhat artificial device that exists in the minds of chemists not within the molecules themselves to help keep track of electrons in their.
Of non - bonding lone pair electrons -. Web Because the bonding pair of electron is shared ie. Calculate the formal charge on the chlorine Cl atom.
One electron is claimed by Cl and one by O this means that the chlorine atom owns 7 valence electrons and is. Web Each Cl atom now has seven electrons assigned to it and the I atom has eight. Web These formal charges can be used to predict the resonance structure that contributes most to the stability of a molecule or ion.
Therefore shape of ion is.

Solved Calculate The Formal Charge Of Chlorine In The Molecules Cl2 Becl2 And Clf5
Solved Calculate The Formal Charge Of Chlorine In The Molecules Cl2 Becl2 And Clf5

Number Of Valence Electrons In Cl Ion Is
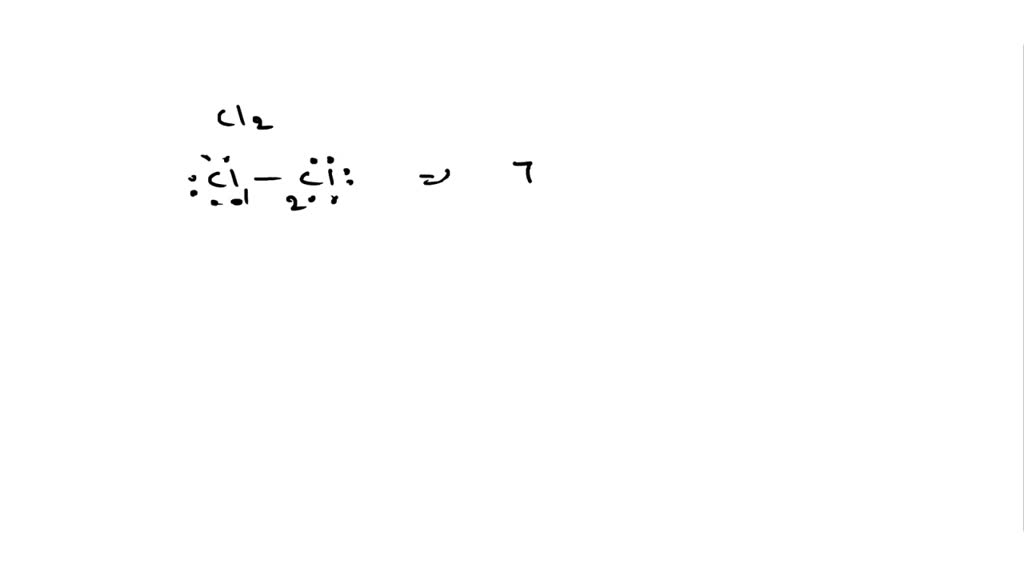
Solved Calculate The Formal Charge Of Chlorine In The Molecules Cl2 Becl2 And Clf5

Illustrating The Bonding Of Sodium And Chlorine Ppt Download

Number Of Valence Electrons In Cl Ion Are Youtube
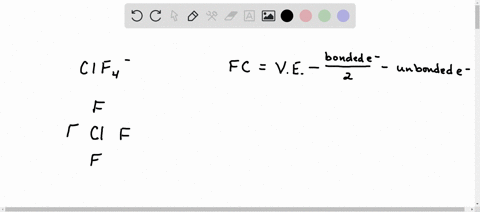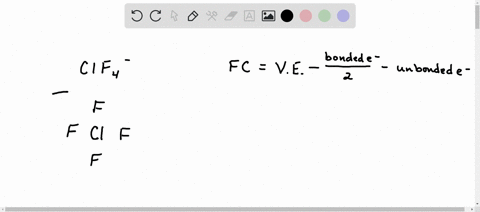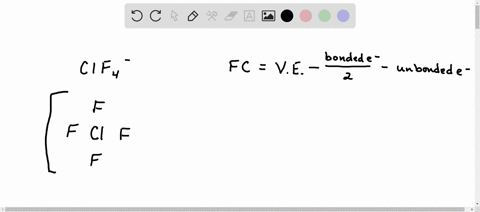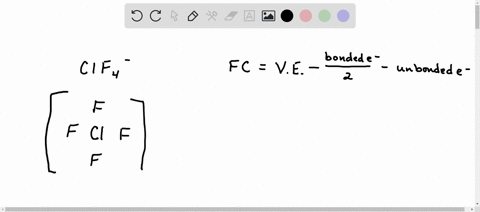
Solved Calculate The Formal Charge Of Chlorine In The Molecules Cl2 Becl2 And Clf5

Covalency Of Trivalent Actinide Ions With Different Donor Ligands Do Density Functional And Multiconfigurational Wavefunction Calculations Corroborate The Observed Breaks Inorganic Chemistry

What Is The Number Of Valence Electron In A Chloride Ion Quora

1 Determine The Formal Charge On The Chlorine Atom In The Molecular Ion Cli 2 1 2 Determine The Formal Charge On The Iodine Atom In The Hio Molecule 3 For The Arsenate Ion

Uranium Iv Chloride Complexes Ucl62 And An Unprecedented U H2o 4cl4 Structural Unit Inorganic Chemistry
Chlorine Cl2 Pubchem

Pdf Chemistry Chrity Grace Academia Edu

What Is The Formal Charge On The Chlorine Atom In The Oxyacid Hoclo2 If It Contains Single Bonds
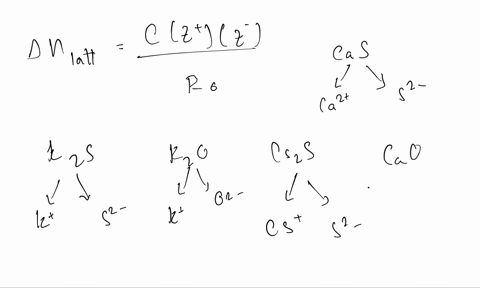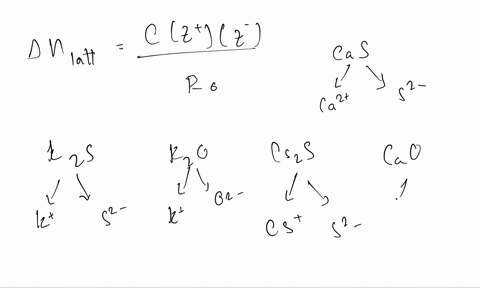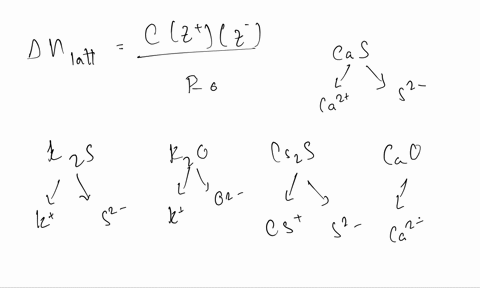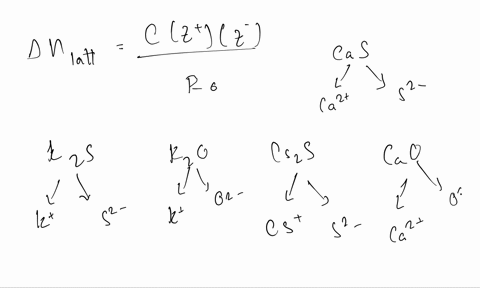
Solved Calculate The Formal Charge Of Chlorine In The Molecules Cl2 Becl2 And Clf5
Why Is Chlorine Ion Said To Be Negatively Charged Quora

Compare Chlorine Atom And Chloride Ion With Respect To Atomic Structure And Electrical State Brainly In