11+ the orbital diagram for a ground state nitrogen atom is
Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. How many orbitals are allowed in a subshell if the angular momentum quantum number.
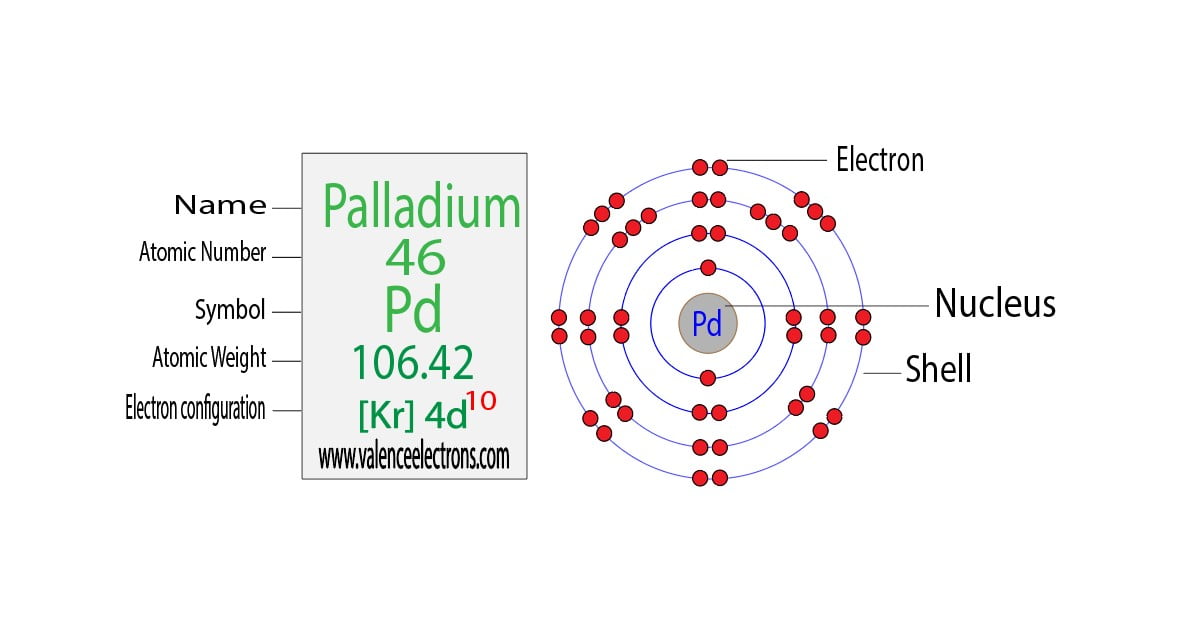
Nitrogen N Electron Configuration And Orbital Diagram
To do that we need to find the number o.

. Nitrogen has a total of 7 electrons and its electron configuration is 1s 2 2s 2 2p 3. What is the orbital diagram for an atom in a ground state. Skip to main content.
To write the orbital diagram for the Nitrogen atom N first we need to write the electron configuration for just N. The orbital diagram for nitrogen is drawn with 3 orbitals. Orbital node crevice pit Node The ground-state electron configuration of a calcium atom is Ne3s2 Ar4s13d1 Ne3s23p6 Ar4s2 Ar3d2 Ar4s2 Consider the element with the electron.
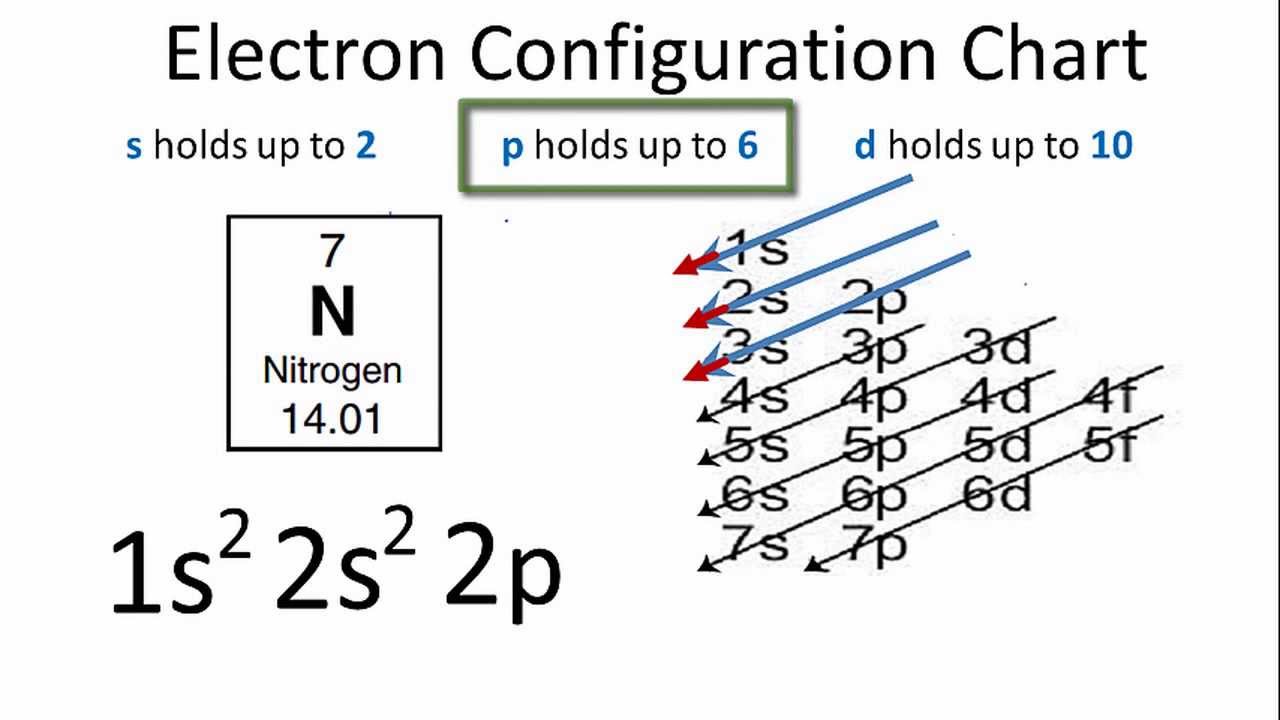
The orbitals are 1s 2s and 2p. C Draw the Lewis structure for CN and. Electronic configuration of nitrogen in ground state is 1s2 2s2 2p3 or 1s2 2s2 2px1 2py1 2pz1.
Hence in excited state one of the 2s electron will jump to 2p orbitalso the excited state. Study with Quizlet and memorize flashcards containing terms like The number of orbitals in a D sun shell is 1 2 3 5 7 1 The orbital diagram for a ground state nitrogen 7 atom is 2 The. 1s 2s 2p 1 L1l B 1 1L I_ c L L11 We dont have your requested question but here is a suggested video that might help.
As per the Aufbau rule the electrons will be filled into 1s orbital first then 2s then 2pso on. The ground state electronic configuration of Nitrogen atom will be 1 s 2 2 s 2 2 p 3 Ground state is the state which has the least energy level with maximum stability. The orbital diagram for a ground-state nitrogen atom is OneClass.
The orbital diagram for a ground-state nitrogen atom is. The nitrogen orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital and. Up to 256 cash back Get the detailed answer.
For example if it was nitrogen it would be clockwise counterclockwise spin i dont know. The orbital diagram for a ground - state nitrogen atom is A. It depends on the atom.
So now we know that. The orbital diagram for ground state nitrogen atom is. This is called quantum jump.
B Give the ground state electron configuration of a carbon atom. A Give the ground state electron configuration of a nitrogen atom. Answer to Solved The orbital diagram for a ground-state nitrogen atom.

Ground State Electronic Configuration Of Nitrogen Atom Can Be Represented By Youtube
The Second Ionization Energy Is Always Greater Than The First Ionization Energy How Is The Second Ionization Of O More Than N But The First Ionization Energy Of N Is More Than
Nitrogen Orbital Diagram Electron Configuration And Valence Electron
Draw The Atomic Structure Of Sodium Atom And Sodiu Class 11 Chemistry Cbse

Magnetic Dipole Lines In Fe Like And Mn Like Molybdenum Ions Sciencedirect

Fine Tuning Of Linear Hexa Cobalt And Defective Penta Cobalt Metal String Complexes Lin 2015 Zeitschrift F 252 R Anorganische Und Allgemeine Chemie Wiley Online Library

Pdf Experimental And Theoretical Study Of Visible Transitions In Promethium Like Tungsten

Exam 3 Study Guide Flashcards Quizlet
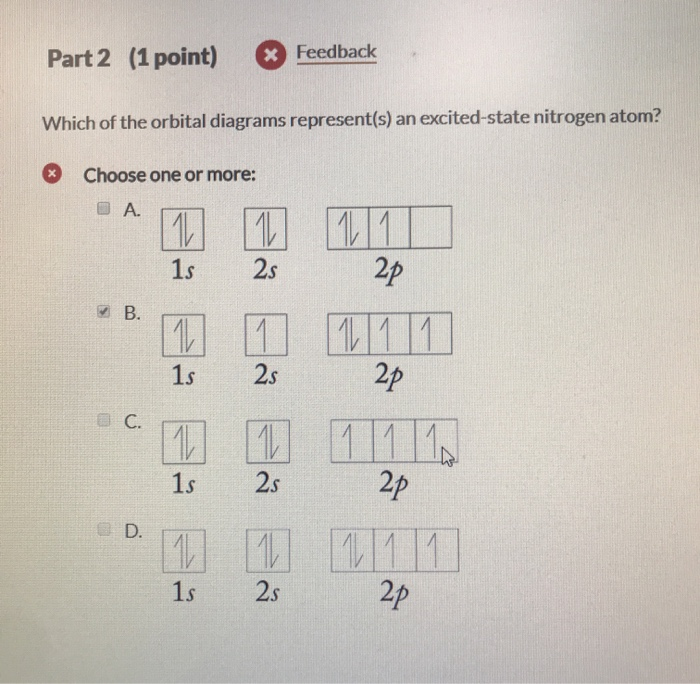
Solved Part 2 1 Point Feedback Which Of The Orbital Chegg Com

Molecular Orbital Diagram Wikiwand
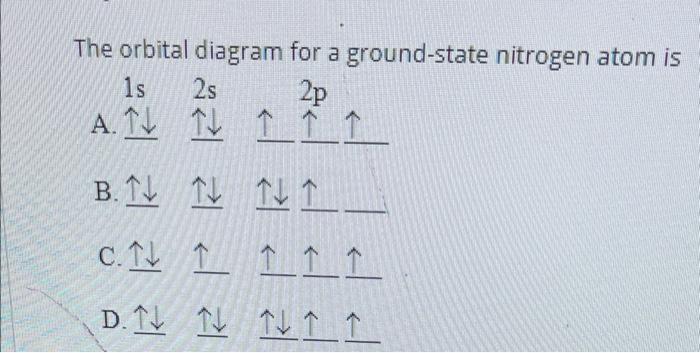
Solved The Orbital Diagram For A Ground State Nitrogen Atom Chegg Com

Nitrogen Electron Configuration N With Orbital Diagram
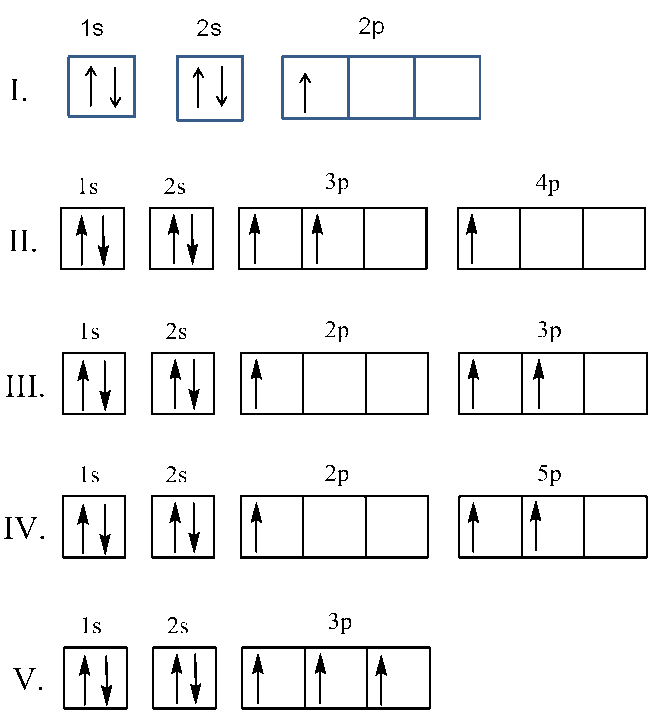
Solved Which Of The Following Orbital Energy Level Diagrams Chegg Com

Nitrogen Orbital Diagram Electron Configuration And Valence Electron
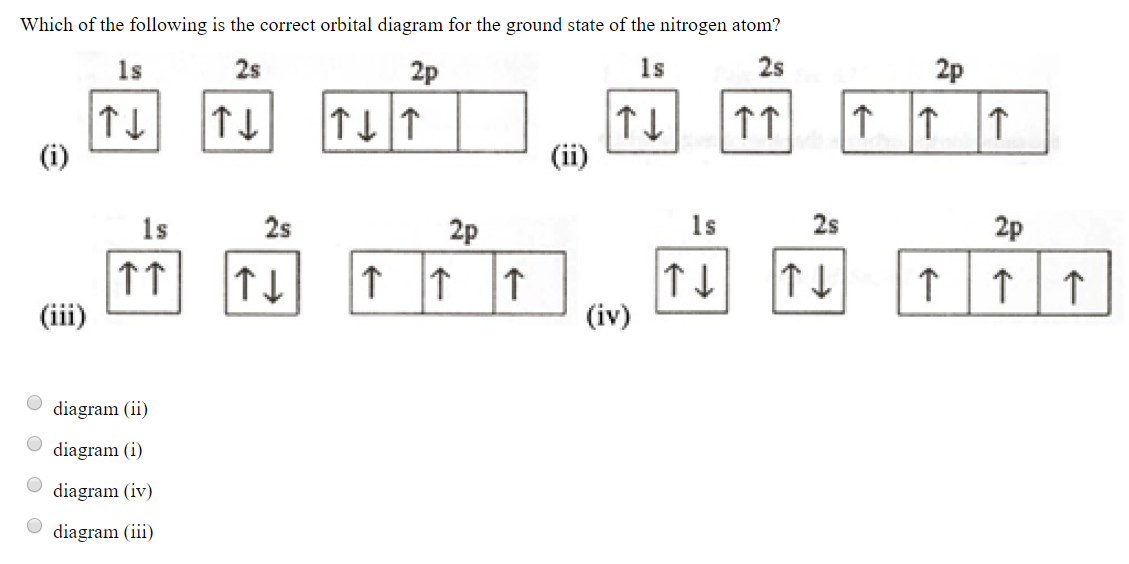
Solved Which Of The Following Is The Correct Orbital Diagram Chegg Com
Solved Write The Orbital Diagram For The Ground State Nitrogen Atom Course Hero
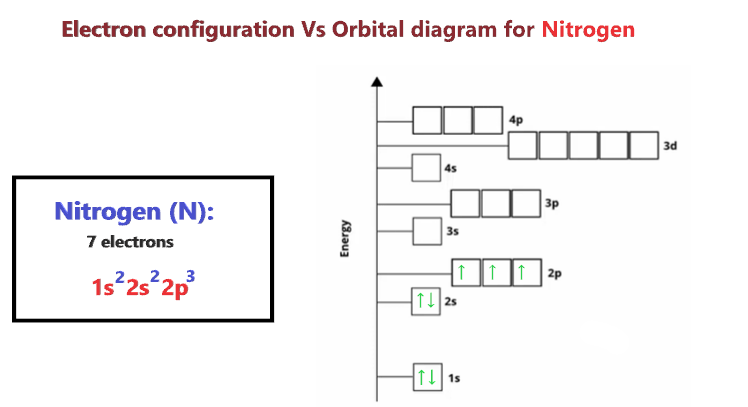
Nitrogen Orbital Diagram Electron Configuration And Valence Electron